Hot-dip Galvanizing Process
Hot-dip galvanizing is the process of immersing iron or steel in a bath of molten zinc to produce a corrosion resistant, multi-layered coating of zinc-iron alloy and zinc metal. While the steel is immersed in the zinc, a metallurgical reaction occurs between the iron in the steel and the molten zinc. This reaction is a diffusion process, so the coating forms perpendicular to all surfaces creating a uniform thickness throughout the part.
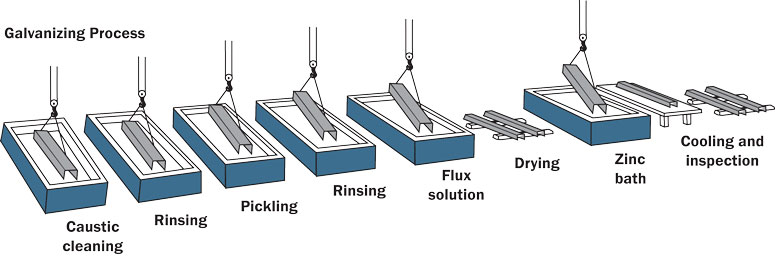
Figure 1: Model of the Hot-Dip Galvanizing Process
Cold galvanizing process
The cold galvanizing technique is different from all the other galvanization types, since it’s an actual painting process. Through this procedure, a resin-based, zinc enriched varnish is applied. Preparations for optimal cold galvanizing occur with a primary excision of calamine (if there is any), degreasing of the substrate and dusting off the surface.
This process is widespread and effectively used under difficult climatic conditions such as marine and industrial environments. Cold galvanizing, among other galvanization types, does not reach the same levels of protection as hot dip galvanization, but it can be employed as a base to make the most of the metal’s antioxidant properties.Then, a second hand of colored finishing polish gets spread out.
The applying of the protective film occurs by way of high electrolysis and allows some advantages, including the possibility to overlay it on very thin or holed products without deforming or obstructing them.
There are additional cold galvanization types like, for example, electrolytically done cold galvanizing process. As opposed to cold galvanizing, this kind of processing is obtained by immersion in acid or alkaline baths charged by an electrical current.
Electro galvanizing galvanization types and procedures
Electro galvanizing is one of the best known galvanization treatment types, ideal for protecting smaller artifacts from corrosion. This particular process guarantees a top quality electro-coated film as an alternative to more expensive methods such as chrome and nickel plating. The purpose of Electro galvanizing is to safeguard the articles from the weather factors’ corrosive action. It occurs through electro-deposition, the steel’s surface is protected by applying a metal layer that technically resembles more a cataphoresis paint than a galvanizing process.
Among the so called galvanization types, the most widely used process is denominated as hot dip bathing, and it is carried out by immersing a ferrous artifact in a tank containing molten zinc at a 450°C temperature. The covering mineral creates a metallurgical reaction with the based one, creating an outer alloy that provides a long-lasting protection. That is not all, it also acts with the electrochemical mechanism: in case the external patina gets damaged, the obtained level would tend to “sacrifice” itself to avoid iron oxidation and consequently rust formation.
Surface Preparation
The purpose of surface preparation in the hot-dip galvanizing process is to obtain the cleanest possible steel surface by removing all of the oxides and other contaminating residues. Thorough surface preparation is paramount as zinc will not react with unclean steel. In order to move the steel parts through the cleaning steps and galvanizing bath, the articles are hung using chains, wires, or specially designed dipping racks (Figure 3).
Cleaning steel to prepare for the hot-dip galvanized coating consists of three steps:
Degreasing
First the steel is immersed in a degreasing bath such as an alkaline caustic solution to remove organic contaminants such as dirt, oil, and grease from the surface of the steel. After degreasing the steel is rinsed with water.
Pickling
Next the steel is pickled in a dilute solution of either hydrochloric or sulfuric acid (Figure 4), which removes oxides and mill scale. Once all oxidation has been removed from the steel, it is again rinsed with water and sent to the final step of the surface preparation.
Fluxing
Finally, the steel is dipped in the flux. The purpose of the flux is to clean the steel of all oxidation developed since the pickling of the steel and to create a protective coating to prevent any oxidation before entering the galvanizing kettle. One type of flux is contained in a separate tank, is slightly acidic, and contains a combination of zinc chloride and ammonium chloride. Another type of flux, top flux, floats on top of the liquid zinc in the galvanizing kettle, but serves the same purpose.
After degreasing, pickling, and fluxing, the surface of the steel is a near white metal, clean and completely free of any oxides or other contaminants that might inhibit the reaction of the iron and molten zinc in the galvanizing kettle.

